Please wait...



Our ocean and coastal waterways are essential to our national security, international trade, maritime commerce, global competitiveness, and transportation. The jobs of more than 3 million Americans depend on our ocean economy, which generates more than $300 billion of economic activity annually. During National Ocean Month, we reaffirm our commitment to responsible stewardship of our ocean resources to strengthen and expand economic opportunities, while also ensuring that the natural beauty and wonder of the oceans are preserved and maintained for future generations.1
Why should we care about the ocean?
Our ocean provides countless benefits to our planet and all the creatures that live here.2
1. Proclamation on National Ocean Month, 2020
2. Ocean Facts, Our Ocean World, National Oceanic and Atmospheric Administration (NOAA), U.S. Department of Commerce
https://oceanservice.noaa.gov/facts/why-care-about-ocean.html




Flavor is the sensory impression of substances like food or beverages. It is a combination of the sensory input of smell and taste with some input from pressure and temperature. The flavor of products can be changed with the addition of flavorants. A “flavorant” is a substance that gives another substance flavor, altering the characteristics of the original substance.
Flavorants can either be natural or artificial. Many natural flavors are very costly so replacement artificial flavorants have been developed. Most commercial flavorants are chemically similar or equivalent to natural flavors which have been synthesized rather than extracted. Different classes of compounds are distinct for their flavor characteristics.
Flavorants for food, beverage and tobacco products are often regulated by government agencies which produce lists of acceptable flavorants. In cases of new products, time can pass between introduction of flavorants before a final decision is made as to their status and safety. Many flavorants for new products in the tobacco and vaping industry can fall under this limbo between legal status and common use.
SPEX CertiPrep offers an extensive catalog of flavorant standards for use in the alcohol, tobacco and vaping industries.
Check out our new Alcohol and Tobacco Brochure
Contact is with any questions at [email protected] or 732.549.7144




Marie Curie, born on November 7th, 1867, was a physicist, chemist and a pioneer in the study of radiation. She worked extensively with radium throughout her lifetime, characterizing its various properties and investigating its therapeutic potential.
In 1883, at the age of 15, Curie completed her secondary education, graduating first in her class. Curie and her older sister, Bronya, both wished to pursue a higher education, but the University of Warsaw did not accept women. To get the education they desired, they had to leave the country. At the age of 17, Curie became a governess to help pay for her sister’s attendance at medical school in Paris. Curie continued studying on her own and eventually set off for Paris in November 1891. Curie was a focused and diligent student, and was at the top of her class. In recognition of her talents, she was awarded the Alexandrovitch Scholarship for Polish students studying abroad. The scholarship helped Curie pay for the classes needed to complete her degrees, in physics and mathematical sciences in 1894.
Upon furthering her education, her curiosity led her to reports of German physicist Wilhelm Röntgen’s discovery of X-rays and by French physicist Henri Becquerel’s report of similar “Becquerel rays” emitted by uranium salts. Curie began to test uranium compounds. She experimented with a uranium-rich ore called pitchblende, and found that even with the uranium removed, pitchblende emitted rays that were stronger than those emitted by pure uranium. She suspected that this suggested the presence of an undiscovered element.
In March 1898, Curie documented her findings in a seminal paper, where she coined the term “radioactivity.” Curie made two revolutionary observations in this paper, Goldsmith notes. Curie stated that measuring radioactivity would allow for the discovery of new elements. And, that radioactivity was a property of the atom.
In June 1903, Marie Curie was the first woman in France to defend her doctoral thesis. In November of that year the Curies, together with Henri Becquerel, were named winners of the Nobel Prize in Physics for their contributions to the understanding of “radiation phenomena.” The nominating committee initially objected to including a woman as a Nobel laureate, but Pierre Curie insisted that the original research was his wife’s.
In 1911, Marie was awarded a second Nobel Prize in Chemistry for her discovery of the elements polonium and radium. In honor of the 100-year anniversary of her Nobel award, 2011 was declared the “International Year of Chemistry.”
However, her work with radioactive materials was what ultimately killed her. She died of a blood disease in 1934. The physical and societal aspects of the Curies’ work contributed to shaping the world of the twentieth and twenty-first centuries.
Sources:
https://en.wikipedia.org/wiki/Marie_Curie
https://www.livescience.com/38907-marie-curie-facts-biography.html
https://www.nobelprize.org/prizes/chemistry/1911/marie-curie/facts/




In honor of Women’s History Month, we have interviewed some of our female scientists here at SPEX CertiPrep and we are so excited for you to learn about our amazing team!
Here is a Q&A we had with Christy Greene
Q. What is your role at SPEX?
A. Territory Sales Manager for West Coast and NY
Q. What led you to study science?
A. Taking business and the courses were very interesting, however, after I finished by associates degree and was moving onto my Bachelor of Science I felt my business major was not stimulating. I like challenges. I wanted courses that provided a struggle, something that did not come easy to me.
Q. What was your favorite science class/lab/project/study?
A. Anatomy and Physiology
Q. What female scientist in history do you most admire?
A. Marie Curie

In honor of Women’s History Month, we have interviewed some of our female scientists here at SPEX CertiPrep and we are so excited for you to learn about our amazing team!
Here is a Q&A we had with Katherine Cullinan
Q. What is your role at SPEX?
A. QC manager for the Inorganic Department
Q. What led you to study science?
A. Science was my favorite subject in school because there wasn’t a right answer to recall and write down on a piece of paper. There was a question and a puzzle to solve through exploration of the world around us.
Q. What was your favorite science class/lab/project/study?
A. In 7th grade we got to do class work, making right angle tubes and also capillary tubes. Working with glass is definitely up there as one of the most fun projects I’ve ever done. In high school, we dissolved zinc out from the inside of a penny, just leaving the copper foil. In college, we removed the plasticizers from saran wrap which feel very strange. In my first job, the flash point test for flammable fuels is a fun way to get paid to play with fire. But only here at SPEX do you get to watch neodymium change colors depending on what room it’s in, precipitate arsenic to what my colleague calls “very toxic Yoo-Hoo”, or help dissolve gold!
Q. What female scientist in history do you most admire?
A. Of course Marie Curie, but also the woman who invented Kevlar, Stephanie Kwolek.

In honor of Women’s History Month, we have interviewed some of our female scientists here at SPEX CertiPrep and we are so excited for you to learn about our amazing team!
Here is a Q&A we had with Patricia Atkins
Q. What is your role at SPEX?
A. Senior Application Scientist
Q. What led you to study science?
A. I was always questioning things and trying to figure out how things worked and science was part of figuring out the answers
Q. What was your favorite science class/lab/project/study?
A. Marine Biology and Zoology Classes
Q. What female scientist in history do you most admire?
A. Rosalind Franklin

In honor of Women’s History Month, we have interviewed some of our female scientists here at SPEX CertiPrep and we are so excited for you to learn about our amazing team!
Here is a Q&A we had with Rebekah Biermann
Q. What is your role at SPEX?
A. Territory Manager (Midwest/New England)
Q. What led you to study science?
A. After years of working in the cosmetics industry, I started to look into cosmetic formulation and went back to school for chemistry. It turns or I didn’t really like formulation chemistry and got a taste for plant genetics and pathology after working on a strawberry research farm at Rutgers University. I learned so much about how agriculture and science crosses over and just how it effects our daily lives is really fascinating.
Q. What was your favorite science class/lab/project/study?
A. Virology and Genetics
Q. What female scientist in history do you most admire?
A. Rosalind Franklin and Barbara McClintock

Q. What is your role at SPEX?
A. Customer Support Manager
Q. What led you to study science?
A. It is something I understood easily and I loved being in the lab
Q. What was your favorite science class/lab/project/study?
A. Chemistry Lab – my first experiment in college was to create crystals and I had the largest cluster with amazing color

In honor of Women’s History Month, we have interviewed some of our female scientists here at SPEX CertiPrep and we are so excited for you to learn about our amazing team!
Here is a Q&A we had with Suzanne Lepore
Q. What is your role at SPEX?
A. Key accounts manager for all SPEX CertiPrep products
Q. What led you to study science?
A. Great teacher in high school
Q. What was your favorite science class/lab/project/study?
A. Analytical Chemistry
Q. What female scientist in history do you most admire?
A. Marie Curie

Join SPEX CertiPrep as we talk about the wide spectrum of Personal Protection Equipment (PPE)
A normal part of laboratory operations is the use of personal protective equipment (PPE). Most laboratory personnel are familiar with common PPE such as gloves, safety glasses and lab coats.
Now, in the time of the pandemic, laboratories are reassessing and adding to their PPE protocols with the addition of face masks, face shields and further protections. This podcast discusses the wide spectrum of disinfectants, sanitizers and additional PPE we are all adding to our laboratory operations.
Hosted by Patricia Atkins, Senior Application Scientist.
Listen to the latest episode

Pumpkin spice season is upon us and our thoughts begin to drift towards the scents and flavors of the season so, we would are happy to share with you our application note, “The Chemistry of Pumpkin Spice“.
Did You Know?
Check Out Our App Note and Infographic for more information on the Chemistry of Pumpkin Spice.


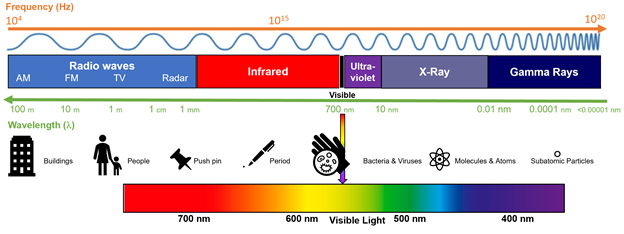
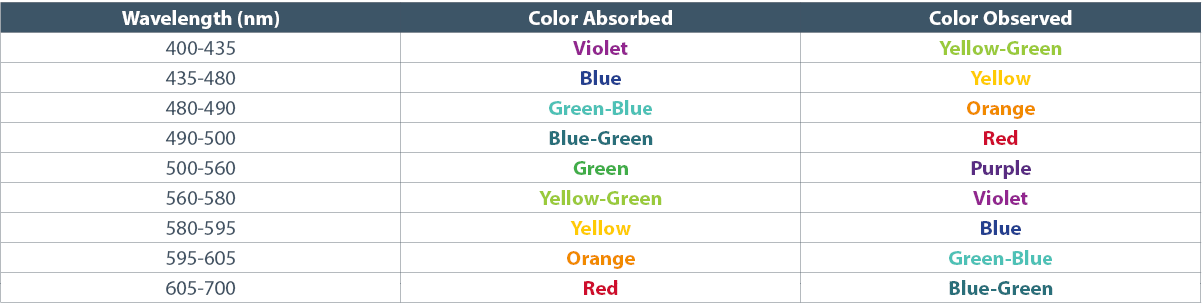
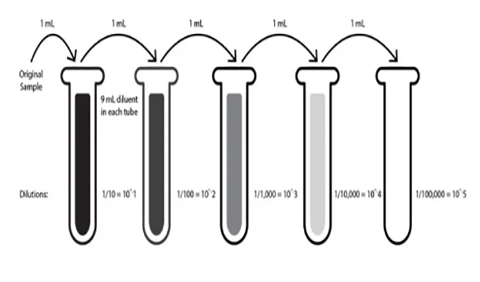

Abstract: The rise of popularity of spicy products and hot sauce has skyrocketed over the last decade. Hot sauces have gone from a few well known brands to a cottage boutique market and an international obsession. In this podcast, we will look at the chemistry of hot peppers and hot sauces and how these products affect the body.
Listen to our Chemistry of Hot Sauce Podcast.

An exploding firework is essentially a number of chemical reactions happening simultaneously or in rapid sequence. When you add some heat, you provide enough activation energy (the energy that kick-starts a chemical reaction) to make solid chemical compounds packed inside the firework combust (burn) with oxygen in the air and convert themselves into other chemicals, releasing smoke and exhaust gases such as carbon dioxide, carbon monoxide, and nitrogen in the process.
Check out our Fireworks Infographic.
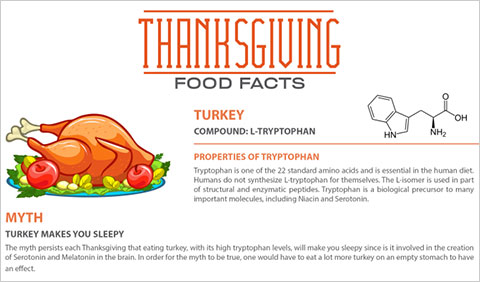
Does turkey really make you sleepy? Check out our Turkey Infographic to find out.

Breast cancer awareness is an effort to raise awareness and reduce the stigma of breast cancer through education on symptoms and treatment. At SPEX CertiPrep, we support the hope that greater knowledge will lead to earlier detection of breast cancer, which is associated with higher long-term survival rates, and that money raised for breast cancer will produce a reliable, permanent cure.
Did you know?
Source: BreastCancer.org https://bit.ly/2NhthCS

Our Senior Application Scientist, Patricia Atkins, recently sat down with Cannabis Science and Technology for a 2 part interview discussing the latest developments in cannabis science.
In part 1, Patricia Atkins discusses her work using pressurized fluid and dispersive solid extraction to maximize analytical cannabis extractions and sample clean-up. You can view part 1 here.
In part 2, Patricia Atkins discusses the next steps in her Cannabis-related research and what excites her most in the field. You can view part 2 here.

September is Prostate Cancer Awareness month. Prostate cancer is the most common type of cancer among men in the United States, other than skin cancer. Prostate cancer affects more than 3 million men every year, worldwide. At SPEX CertiPrep, we support the hope that the greater knowledge will lead to earlier detection for higher long-term survival rates.
During the month of September, all web orders will receive a FREE blue vial rack to brighten up your bench space and be a reminder to take the steps to plan and detect prostate cancer in its early stages and encourage others to do the same. Awareness needs to be ongoing so we are putting blue in your lab!


