Please wait...
Internal Standards for UV-VIS
Patricia L. Atkins • Senior Applications Scientist & Alan H. Katz, PhD • Director, Global Product Manager - Chemicals
| Shop Internal Standards for UV-VIS | Have my Sales Representative Contact Me | Have a technical question? Ask Now |
Ultraviolet-Visible Spectroscopy (UV-VIS) is a frequently used tool in many fields of chemistry. Its use is central in a wide range of techniques, from simple colorimetric assays to complex biomedical experiments. Many organic or inorganic chemicals absorb light in the range of roughly 200-800 nm, depending on their electronic structure. The technique can be used to confirm the identity of a structure, but it is more frequently used to measure the concentration of substances in a mixture.
Each molecule absorbs differently, depending on its extinction coefficient (the measurement of absorption of light in a given medium). Chromophores, such as functional groups with many pi-electrons, absorb more strongly than the saturated analogs. Extinction coefficients are also sensitive to the environment around a given molecule, such as the presence of a protein in binding experiments. One way to simplify the determination of concentration is to include internal standards.
Spex is happy to announce a new line of internal standards for UV-VIS to allow the user to accurately measure the concentration of their samples by directly comparing the absorbance of their compounds against that of the internal standard. We offer 6 different standards that cover a wide range of wavelengths (270 nm - 570 nm). Once the user takes a UV-VIS spectrum of their sample, they can find a region in the spectrum that doesn’t absorb, and then select the corresponding internal standard, and rerun the spectrum. Each internal standard comes with a certificate specifying the exact concentration of each standard.
All 6 standards are organic dyes, which absorb very strongly in the UV-VIS region. This means the standards are very low concentration, which minimizes the possibility that it will hinder solubility of the combination of standard and sample.
These standards are easy to use: 1. Find a spectral region of the sample that is free of peaks. 2. Select one of our internal standards appropriate for that region. 3. Add the IS to your external standards and samples.
Example 1. UV-413W IS was added in increasing aliquots (40-200 μL) and graphed for relative absorbance (Figure 1).
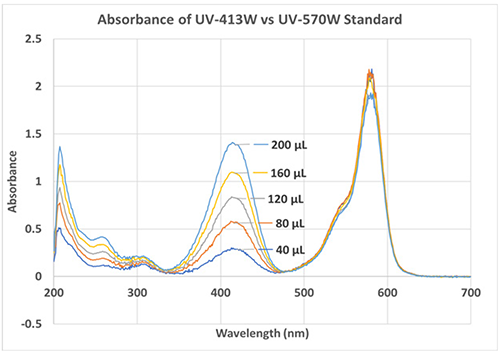
Figure 1. The relative absorbance of each aliquot relative to the amount of UV-413W in solution.
Plotting the 413 absorbance maximum against the volume of standard used is shown to produce a linear curve with > 99.9% correlation. Figure 2a. The data can also be evaluated as a ratio of absorbances such as the ratio in Figure 2b. between 413 and 570 nm (> 99% correlation).
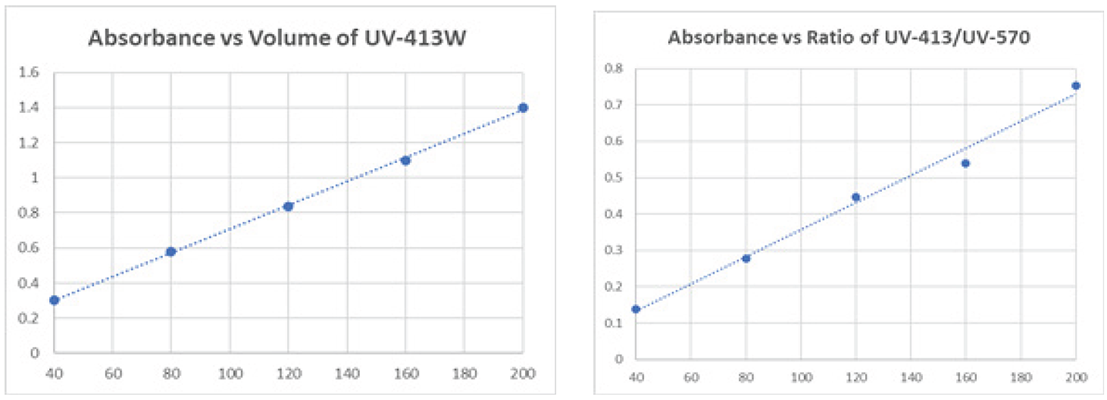
Figure 2. Relative ratios for absorbance plot (a) 413 nM (b) ratio 413/570 nm.Figure 2. Relative ratios for absorbance plot (a) 413 nM (b) ratio 413/570 nm.
This methodology can be extended to kinetic situations. For example, you can study the rate of acid catalyzed decomposition of a sample, by adding a drop of acid, and monitoring the spectrum over time. The decreasing absorbance of the sample compared to the internal standard will allow you to monitor the concentration directly. Similarly, one can mix an inhibitor with a protein, and monitor the rate of inhibitor binding.
The standards are sealed in ampules to reduce exposure and are designed to conveniently fill the standard industry cuvettes (3.5 mL).
This methodology can be extended to kinetic situations. For example, you can study the rate of acid catalyzed decomposition of a sample, by adding a drop of acid, and monitoring the spectrum over time. The decreasing absorbance of the sample compared to the internal standard will allow you to monitor the concentration directly. Similarly, one can mix an inhibitor with a protein, and monitor the rate of inhibitor binding.
The standards are sealed in ampules to reduce exposure and are designed to conveniently fill the standard industry cuvettes (3.5 mL).
| Description | Wavelength | Concentration | Volume | Matrix | Part # |
| 3-chloro-10-[3-(dimethylamino)propy]9(10H)-acridinone hydrochloride | 270 nm | 9.3 ppm | 3.5 mL | Water | UV-270W |
| 7-methoxycoumarin-4-acetic acid | 323 nm | 41 ppm | 3.5 mL | Water | UV-323W |
| Thioflavin T | 413 nm | 18 ppm | 3.5 mL | Water | UV-413W |
| Coumarin 6 | 459 nm | 32 ppm | 3.5 mL | Methanol | UV-459M |
| Eosin Y | 524 nm | 15 ppm | 3.5 mL | Water | UV-524W |
| 3,3’-diethyloxadicarbocyanine iodide | 570 nm | 0.4 ppm | 3.5 mL | Water | UV-570W |
| Download the complete technical note |